Meningococcal B (MenB) vaccine is the new kid on the block for children and adolescents, having won Food and Drug Administration approval in 2014. This presents a problem for physicians: Because there is an older vaccine for the other types of meningococcal bacteria, many patients who’ve had that vaccine wrongly believe they’re also protected against MenB.
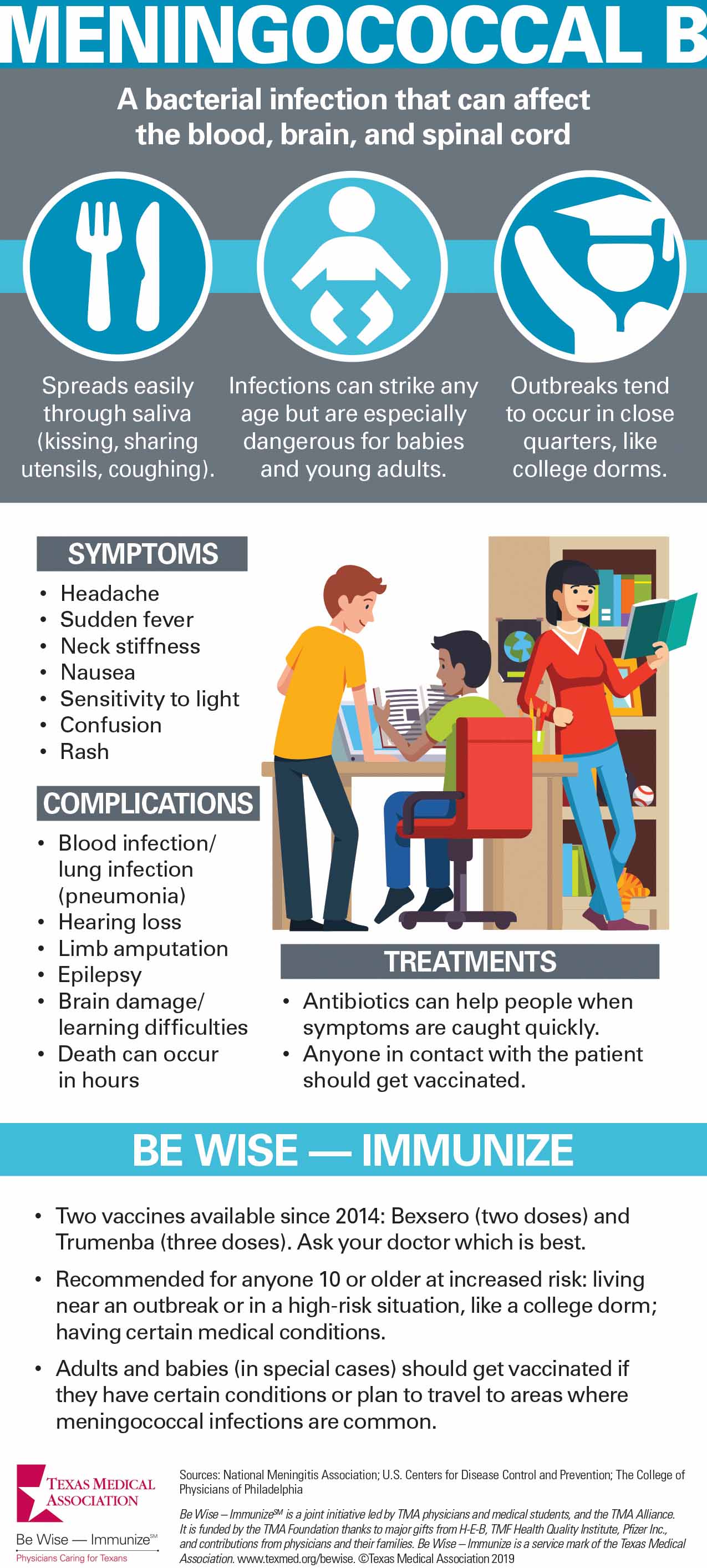
Meningococcal disease remains rare in the U.S., but MenB causes more than half of all cases. This bacterial infection can affect the blood, brain, and spinal cord with lasting effects like learning difficulties, hearing loss, or limb amputation.
MenB vaccine also can be confusing because unlike the other meningococcal vaccine, MenB is not recommended for all young teenagers. Instead, Centers for Disease Control and Prevention (CDC) recommends it for kids 10 and older only if they have certain health problems or are exposed to a disease outbreak. The preferred target age range is 16-18 years. Vaccinating infants, another high-risk group, has not been approved in the U.S., but it is done in some other countries.
CDC also recommends vaccinations for young adults living in close quarters, like college campuses or barracks. Between 2013 and 2018, CDC reported 10 university-based MenB outbreaks in seven states (not including Texas), which caused 39 cases and two deaths.
There are two MenB vaccines: Bexsero and Trumenba. Both require multiple doses; the same brand of vaccine should be used for all doses.
Download a printable copy of the infographic below.
Tex Med. 2019;115(6):47
June 2019 Texas Medicine Contents
Texas Medicine Main Page
Last Updated On
August 02, 2019
Originally Published On
May 20, 2019