The Texas Medical Board (TMB) and Texas Medical Association remind physicians to continue to use strong medical judgment when considering drugs and therapies to treat COVID-19 and to take all appropriate steps when making any treatment decision.
“Both patients and physicians have a right to decide what treatment may be used for COVID-19,” TMB said in a statement. “The board does not issue endorsements of the use of any specific drugs or treatments for COVID-19, but any treatment decision must be made with full, proper and accurate disclosure by a physician.
“While there are drugs and therapies being used to treat COVID-19, there is no definitive cure at this time,” the board continued.
TMA President Diana Fite, MD, encouraged physicians continue to study treatment options and report any successes to health authorities.
“As you know, some medical breakthroughs were originally dismissed as unproven or outlandish but later adopted as the scientific proof mounted,” Dr. Fite said. “We strongly encourage anyone who believes they have an effective pre- or post-exposure treatment for COVID-19 to submit them appropriately through the rigors of clinical testing and peer review.”
Clinical trials are under way for some new medications, while existing drugs also are being tested to see whether they are safe and effective in treating the disease. To do so, the Food and Drug Administration (FDA) has made some drugs available through its Expanded Access Program, or under Emergency Use Authorizations.
Remdesivir, the only FDA-cleared drug to treat COVID-19, has shown some promise in speeding recovery of patients hospitalized with the disease.
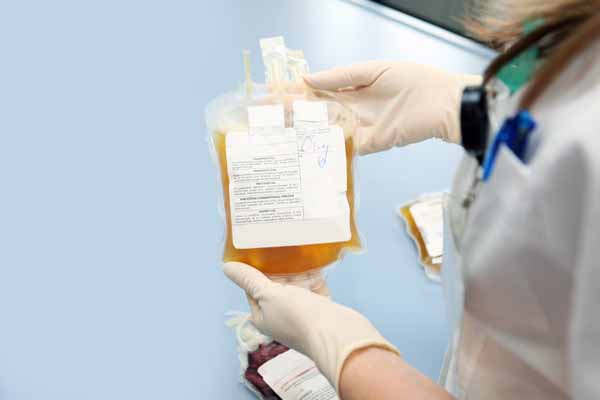
Convalescent plasma also is one of the most promising treatment options being studied. However, medical researchers still have “insufficient data” to recommend either for or against using convalescent plasma to treat COVID-19, according to the National Institutes of Health (NIH) treatment guidelines.
TMB reminded physicians to refer to its rules as well as state and federal laws when considering treatments regarding COVID-19.
“A physician must provide full disclosure of treatment options, side effects, obtain informed consent, and there cannot be false, misleading or deceptive advertising or statements made regarding any therapies, including a cure for COVID-19,” TMB added.
TMB’s disciplinary process is complaint driven. The board statement said it could conduct standard-of-care reviews for COVID-19 treatment complaints and could “investigate complaints for false, misleading or deceptive advertising, which could include assuring a permanent cure for an incurable disease.”
Relying on unproven treatments could create a false sense of protection among patients that might lead to exposure to the disease, Dr. Fite also said.
The TMA COVID-19 Task Force in June updated its frequently asked questions (FAQ) on COVID-19 treatment. The FAQ is based on guidance from NIH, the Centers for Disease Control and Prevention (CDC), Texas Department of State Health Services (DSHS), and other public health organizations.
The Task Force has issued recommendations on only one prospective COVID-19 treatment. Per the NIH COVID-19 Treatment Guidelines, which cites insufficient clinical data, the TMA COVID-19 Task Force recommended on June 19, 2020, “against using chloroquine or hydroxychloroquine to treat COVID-19 except in a clinical trial.”
You can also find the latest news, resources, and government guidance on the coronavirus outbreak by visiting TMA’s COVID-19 Resource Center regularly.